Mixing It Up: An Overview of Upcoming Changes to USP Guidelines
Summary: This article delves into the upcoming updates in the United States Pharmacopoeia (USP) chapters <795> and <797>, effective November 1, 2023.
Table of Contents
USP <795> - Non-Sterile Compounding Updates
USP <797> - Sterile Compounding Updates
Summary
On November 1, 2023, the updated United States Pharmacopoeia (USP) chapters <795> and <797> compounding standards will become effective. How well do you know what’s in the new standards and what this means for your pharmacy? Let’s take a deep dive into this important topic for any pharmacy that engages in sterile or non-sterile compounding.
We are currently a little over halfway through the one-year implementation timeline since the revised USP <795> and <797> standards were published on November 1, 2022. The updated guidelines reflect advancements in the science and practice of compounding, along with stakeholder feedback, and are intended to improve the quality and safety of compounded drugs. On November 1, 2023, the revised chapters will become effective, and many state pharmacy boards have added new guidelines to their respective pharmacy regulations. This article will provide a detailed overview of the new guidelines to help pharmacies comply with adoption of these updated requirements.
Many of the updated guidelines center around Beyond-Use Date (BUD) calculations for compounded sterile and non-sterile preparations. Beyond-Use Dates are the time limits after which a compounded preparation must not be used or kept. After the BUD arrives, the compounded preparation may be at increased risk of microbial contamination, degradation, or loss of container closure system integrity. The BUD is calculated based on the date and time the compound is prepared. Assigning and complying with clinically appropriate BUDs is necessary to ensure patient safety.
USP <795> - Non-Sterile Compounding
Non-sterile compounding is more than just coal tar ointments and magic mouthwash – USP <795> also covers commercially available compounding kits (ex. erythromycin/benzoyl peroxide gel) and flavoring agents like FlavoRx. However, non-sterile compounding does not include tablet splitting, or reconstituting antibiotic suspensions.
The USP <795> updates include:
Revised training guidelines to ensure pharmacy staff can appropriately and safely perform necessary compounding activities
New schedules for cleaning and sanitizing surfaces in the compounding area
Revisions to Beyond-Use Date (BUD) tables and calculations – including clarified guidance on assigning BUD extensions beyond the default timeframes based on water activity. Water activity is a new concept that is used in determining the potential for contamination or degradation in compounded non-sterile products (CSNPs)
Pharmacies will need to ensure that standard operating procedures (SOPs) are in place and that training is documented. Enforcement of the new guidelines will vary by state. Check with your state board of pharmacy to determine how the updates will be implemented and enforced.
USP <797> - Sterile Compounding
Sterile compounding involves the preparation of compounded sterile products (CSPs), often for intravenous or parenteral use. Sterile compounding requires strict engineering and environmental controls to ensure CSPs are free from contamination.
The USP <797> updates include:
Enhancements to cleaning and sanitization requirements, including the use of sporicidal agents
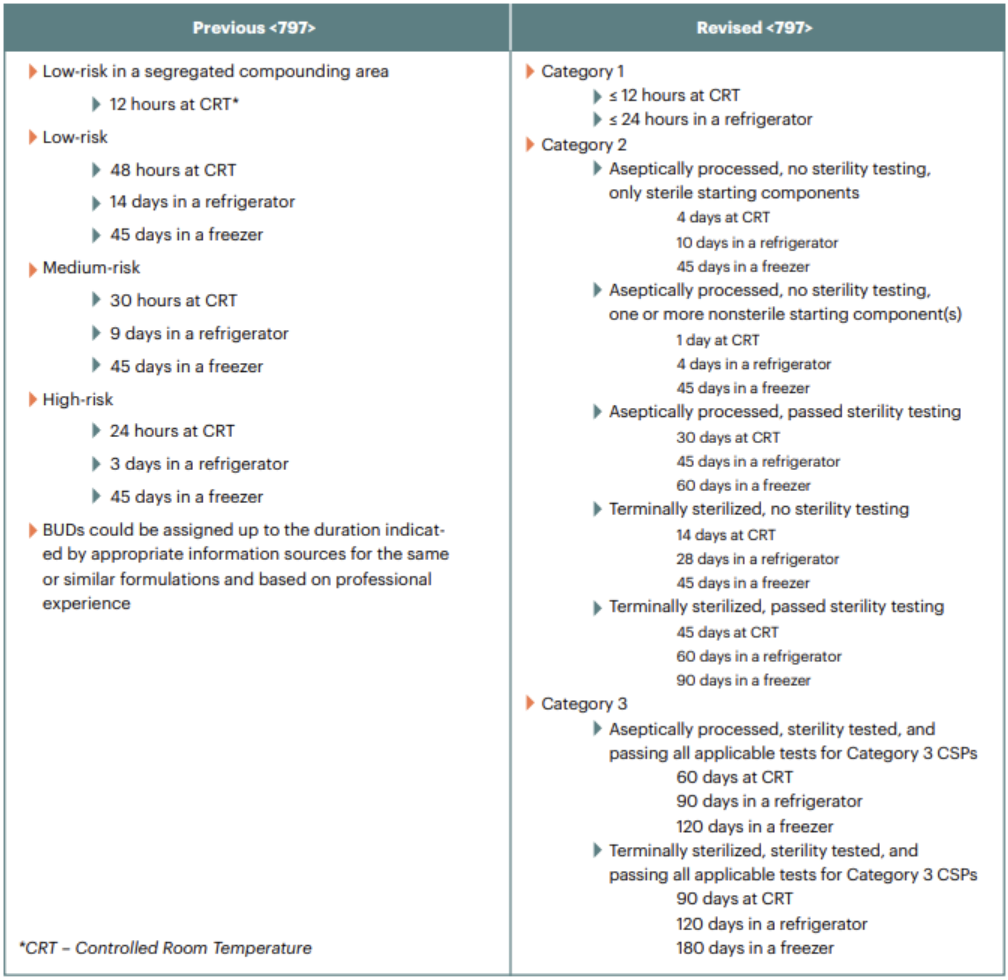
A complete revision of CSP categorization.
The previous guidelines differentiated sterile compounding activities based on the risk of microbial contamination (low-, medium- and high-risk preparations). These classifications have been replaced by a category system that is based on the environmental conditions under which the CSPs are made. Category 1 CSPs are those made in an ISO Class 5 hood or incubator outside of a clean room suite and have shorter BUDs. Class 2 CSPs are prepared in a clean room and have longer BUDs. The addition of a new category of CSP (Category 3) was also added to provide guidance for CSPs with a BUD of up to 180 days.
Each category has its own requirements for garbing, cleaning/sanitizing, environmental monitoring frequency, and personnel testing that must be adhered to. Pharmacy staff involved in compounded CSPs can expect to perform more frequent evaluations for media fill testing, fingertip sampling, cleaning, garbing, calculations, and other cleanroom procedures.
Summary
Now is the time to ensure training, staff competency, policy/SOPs, quality measures, environmental monitoring procedures, and primary engineering controls are in compliance with the new standards.
If your pharmacy prepares sterile or nonsterile compounds, compliance is a major concern. Many state boards are preparing to monitor and enforce the updated USP <795> and <797> guidelines. While the updated guidance will become effective on November 1, 2023 – state pharmacy boards may choose a different date for enforcement. The Joint Commission plans to enforce them beginning January 1, 2024. Pharmacies should consult their state board of pharmacy to determine requirements and timelines for enforcement.
References:
https://go.usp.org/USP_Compounding_BUD_Fact_Sheet.pdf
https://www.fda.gov/drugs/guidance-compliance-regulatory-information/human-drug-compounding
USP <795> FAQ: http://go.usp.org/USP_GC_795_FAQs
USP <797> FAQ: http://go.usp.org/USP_GC_797_FAQs